Wat is Added First the Continuous Phase of the Dispersed Phase
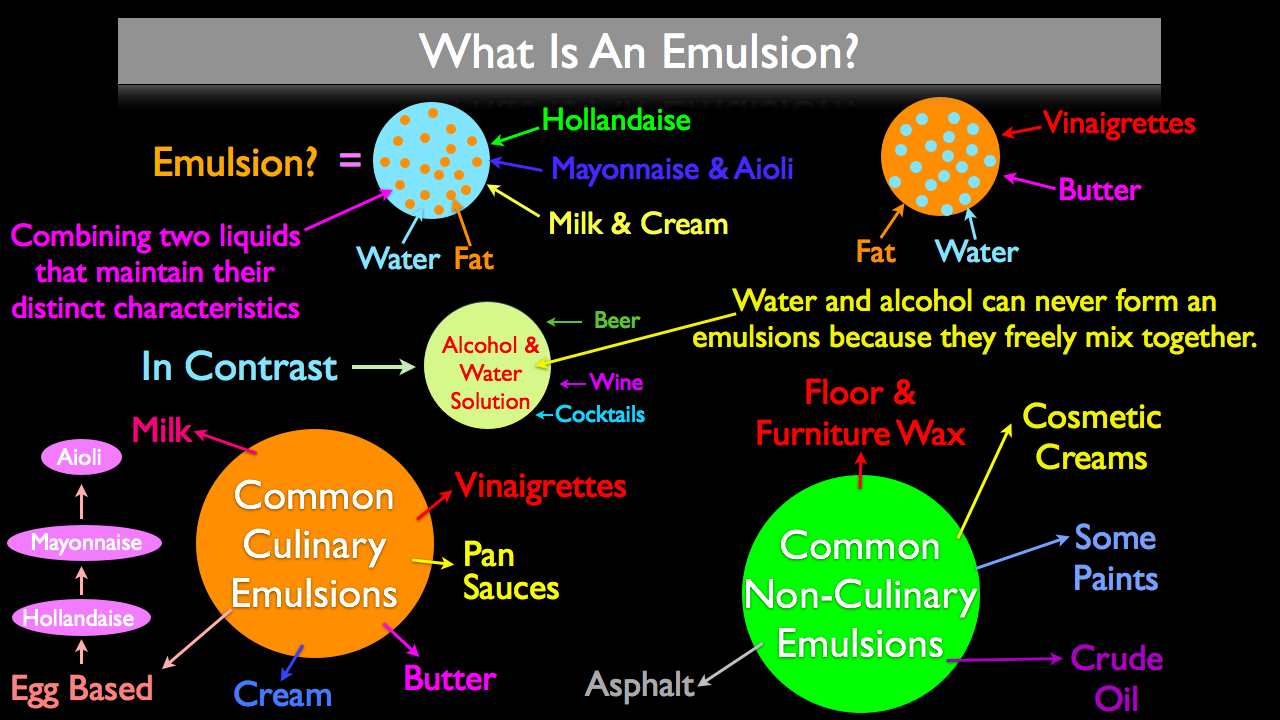
What Is An Emulsion?
An emulsion is defined by combining two liquids that will maintain their distinct characteristics after being mixed. When talking about emulsions as applicable in a kitchen, the term emulsion refers to combining fat and water. Culinary emulsion can take two different forms; fat dispersed into water and water dispersed into fat. Common fat in water emulsifications include hollandaise, mayonnaise, aioli, milk, cream, and pan sauces. Water in fat emulsifications are most commonly found in the form of vinaigrettes and whole butter.
In contrast to water and fat maintaining their distinct characteristics when combined, other water friendly liquids like alcohol can never form an emulsion with water because of their ability to freely mix. This is a good thing; if alcohol reacted to water like fat does, then that nice bottle of wine would be nothing more then grape juice with pure alcohol floating on top.
Understanding Emulsions & How They Work
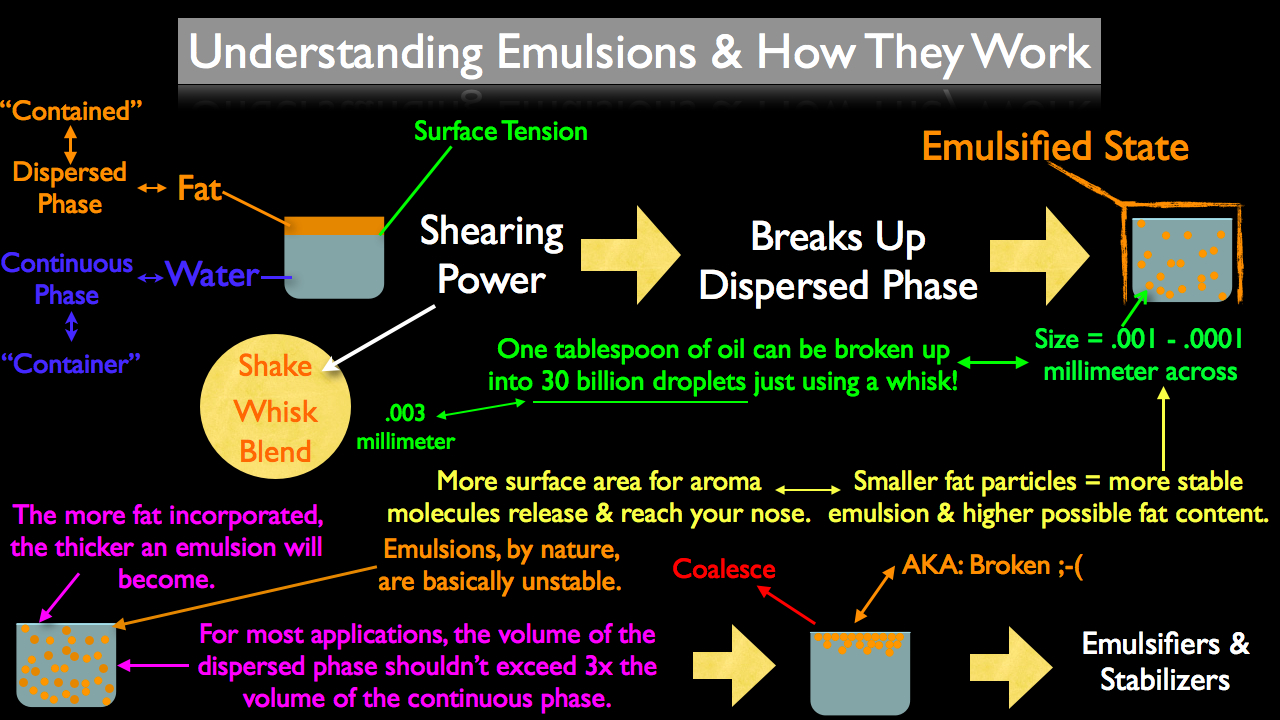
In scientific terms, fat and water are considered "immiscible," or two liquids that don't like to mix. Chemically speaking, when two molecules don't freely mix they will align themselves in such a fashion to touch as little as possible. This alignment is described as "surface tension." It's this surface tension the cook will constantly battle when attempting to make and maintain an emulsification.
In any emulsification there are two phases, the continuous phase and the dispersed phase. The continuous phase can be thought of as the "container" of the emulsification; it is what the other liquid will be "dispersed" into. It's important to keep track of which phase is which because adding more of the continuous phase will thin an emulsion and more of the dispersed phased will thicken an emulsion.
So how do cooks combine fat and water even though they don't like to mix? By using shearing power.
Shearing power can be described as a liquid in motion; this can range from shaking, stirring, blending, etc. Shearing power is necessary in the emulsification process to break up the dispersed phase and distribute it throughout the continuous phase. The more shearing power used, the smaller the dispersed phase will become, creating a more stable emulsion. The smaller the dispersed phase, the more of that liquid you can add, yielding a thicker emulsion.
However, this can be taken too far. Like we talked about above, the more dispersed phase contained within an emulsion, the thicker the emulsion will become. But as more of the dispersed phase is added, the dispersed particles become crammed closer together. Eventually there is so much of the dispersed phased that it becomes impossible to keep the individual particles separated. This leads to the dispersed phase first stating to touch, combining, pooling and finally re-creating the natural surface tension. This recreation of surface tension as it applies to cooks is commonly referred to as "broken." For most culinary emulsions, the dispersed phase should not exceed three times the volume of the continuous phase.
Adding too much of the dispersed phase to a given emulsion is only one of many reasons an emulsification will "break." In fact, emulsifications are unstable by nature. Water and oil will always attempt to re-create their natural surface tension, but this inevitability can be prevented by using emulsifiers and stabilizers.
Understanding Emulsifiers and Stabilizers
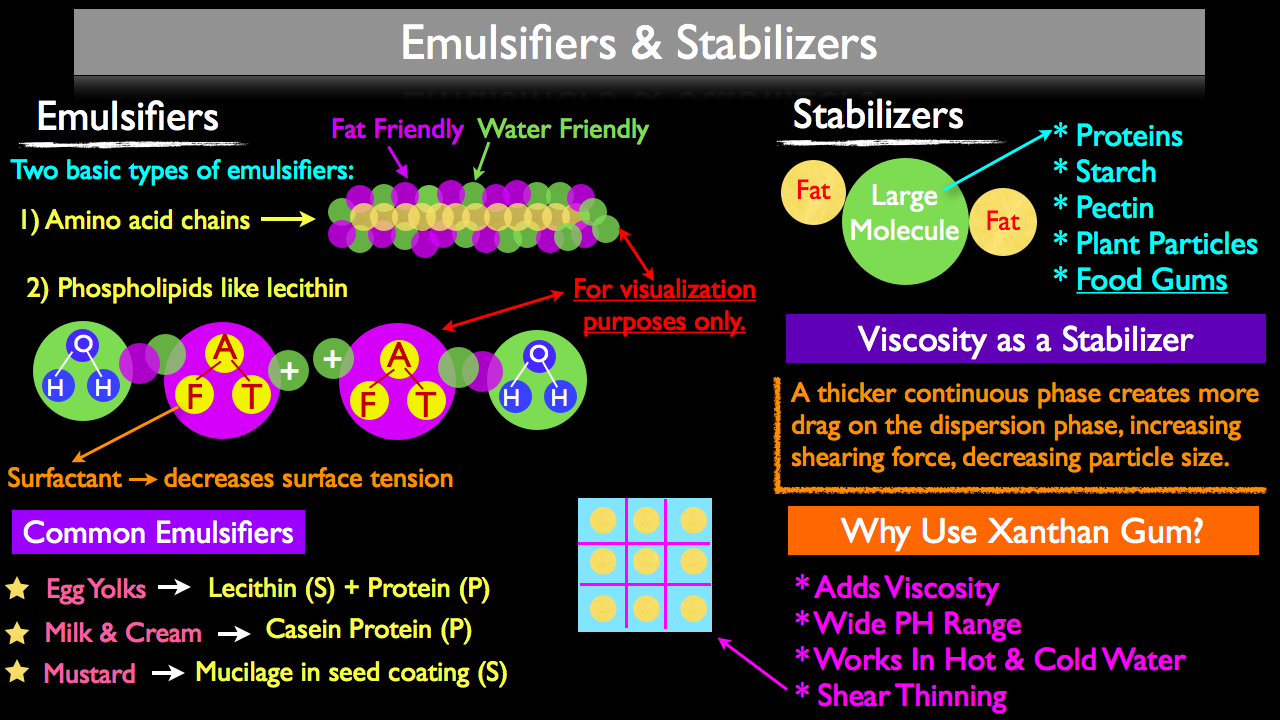
Emulsifiers and stabilizers are commonly used interchangeably in general kitchen conversations because they both serve the same end goal; stabilizing an emulsion. Emulsifiers however are different from stabilizers and each serves a unique purpose depending on your desired finished product.
There two basic types of emulsifiers a cook can use when creating an emulsion; amino acid chains and phospholipids.
Amino acid chains will link together, forming larger chains that we know as proteins. Some of these amino acid chains have both fat friendly and water friendly receptors. A good example are the amino acid chains that make up casein protein, commonly found in egg yolks and milk. Since these amino acid chains by nature are capable of linking fat and water together, by definition they are an emulsifier.
The second form of emulsifiers used in kitchens are phospholipids like lecithin, commonly found in egg yolks and soy. Lecithin is classified as a "surfactant," meaning it has a water friendly head and a fat friendly tail. Lecithin also has the added benefit of have a positively charged tail which makes it an extremely powerful emulsifier, especially when used in a fat-in-water emulsion. The positively charged tail will cause the dispersed fat to actually repel one another, preventing the fat from pooling or "coalescing," which is usually the first step in an emulsion breaking.
Whereas emulsifiers will actually link fat and water together, stabilizers are large molecules that will get in between individual particles contained within the dispersed phase, keeping them from coalescing (pooling together). These "large molecules" can take the form of proteins, starch, pectin, plant particles and food grade gums (a favorite of modern chefs). Because most of these molecules are water soluble, they will usually be utilized with fat-in-water emulsifications.
Inseparable from the concept of large molecules being used as stabilizers is the fact that these large molecules will also "thicken" or add viscosity to an emulsion. In fact, viscosity can help achieve a more stable emulsion especially when starting with a more viscous continuous phase. This is often the case, since food grade gums, starches, plant particles (think purees) will be added to part of the continuous phase before the dispersed phase (commonly fat) is added to the emulsion. A thicker or more viscous continuous phase will create more drag on the dispersed phase, increasing shearing power, effectively decreasing the particle size of the dispersed phase. As we discussed before, the smaller your particle size, the more stable your emulsion. When using stabilizers you have the added benefit of these large molecules that first aided in forming a more stable emulsion by facilitating a smaller dispersed phase, now getting in the way of the dispersed phase, preventing it from coalescing and braking the emulsion.
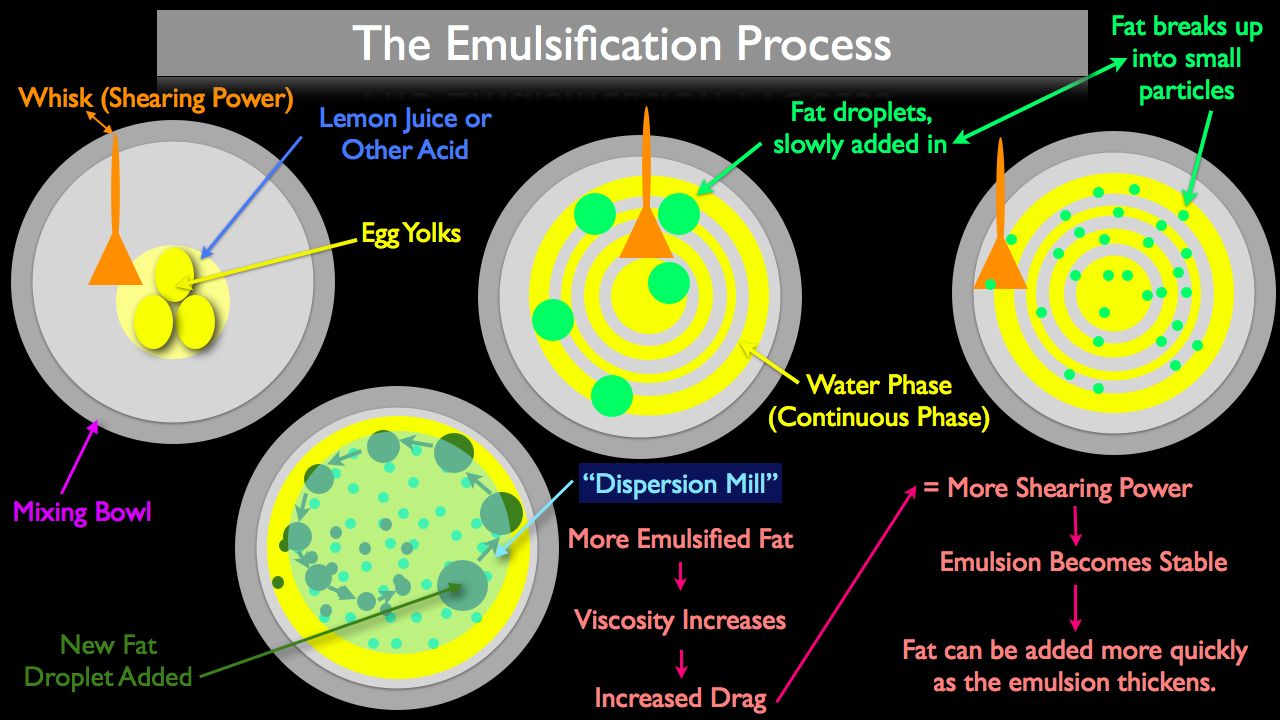
How the Emulsification Process Works
In the video above and the picture below, you can see the basic emulsification process as it relates to making mayonnaise. This particular emulsification is started by placing acid (commonly lemon juice) and egg yolks in a metal mixing bowl. Shearing power is applied in the form of stirring the contents of the bowl with a whisk.
The egg yolk and lemon juice in this case will be the continuous phase, (the emulsion's "container"), to which we will add our fat (the dispersed phase). It's important in the early stages of the emulsification process to add a very small amount of fat slowly at first, usually just a few drops at a time. The reason this slow start is important is because in the early phases of the emulsification process, the fat has plenty of room to freely move around, avoiding the whisk and instead just floating on top of the water as it naturally prefers to do. Once the fat starts to break up and becomes dispersed throughout the emulsion, additional fat can be added more quickly because the already dispersed droplets will work much like a "mill," breaking the dispersed droplets up into smaller particles, making it much easier to incorporate the dispersed phase in a more continuous stream.
It's important to note however that the more shearing power you are using, the less important this "dispersion mill" becomes. It's much harder for a fat droplet to escape the rapidly churning blade of a high speed blender then that of a hand powered whisk.
To give you a general reference point, the chart below lists some of the most common forms of culinary emulsions while defining both the continuous phase, dispersed phase, emulsifier used, water-emulsifier-fat ratio and shelf stability at normal serving temperatures. All ratios are calculated by weight.
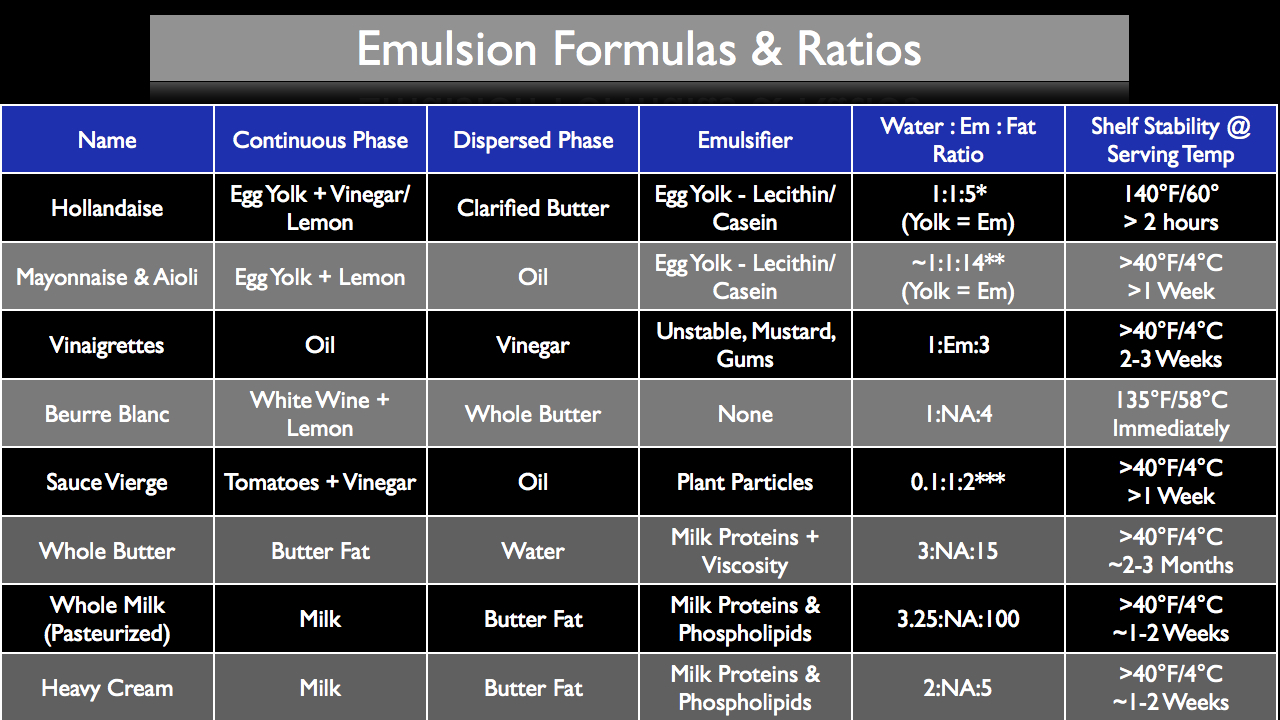
*/**Even though technically the emulsifier is the lecithin and casein contained in the egg yolk, the whole yolk by weight is considered to be the "emulsifier" in this equation to make calculations easier. This means that if you are making a hollandaise sauce and you have 20g lemon juice, you would add to that 20g egg yolks and 100g clarified butter.
***Sauce Vierge is a good example of using plant particle and a viscous continuous phase as a stabilizer. In this ratio, the plant particles (tomatoes) are considered the emulsifier for ease of calculation. That means your for every 10g of vinegar or water, you would add 100g plant tissue (tomatoes) and 200g oil. In the linked recipe below, Xanthan Gum is also used as an optional "insurance policy" to make the overall recipe more forgiving.
Source: https://stellaculinary.com/cooking-videos/food-science-101/fs-001-what-emulsion-cooks-guide
0 Response to "Wat is Added First the Continuous Phase of the Dispersed Phase"
Post a Comment